How Surgical Masks Production are Made?
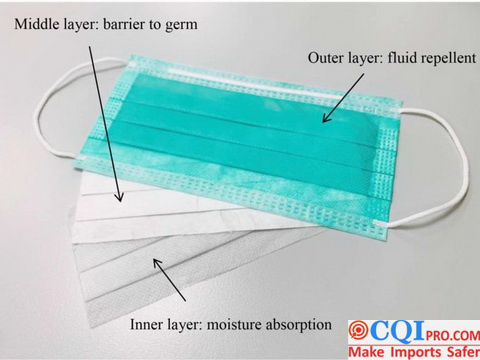
Common Surgical Masks are mainly composed of three layers of non-woven fabrics. The inner layer is a common non-woven fabric; The outer layer is waterproof non-woven fabric, which is mainly used to isolate the liquid ejected by patients. The filter layer in the middle is polypropylene melt-blown nonwoven fabric treated by electret process. Finally, after the completion of surgical mask production , it shall be disinfected by ethylene oxide, allowed to stand for 7 days to volatilize toxicity, sealed, packed and transported away. The core material of surgical mask is melt-blown cloth after electret treatment. Please remember these two key words, because this is the key to filter the new Coronavirus aerosol.
Here we briefly analyze the transformation process from polypropylene particles (PP) to masks, how surgical masks production are made ?
1.Raw materials of surgical mask production : polypropylene (PP) particles to polypropylene fiber production.
The production of non-woven fabrics needs fiber materials. Polypropylene particles are used to melt and shape, and polypropylene fiber material is the core raw material for masks, namely, the polypropylene fibers in strips as shown in the figure below.

2. Melt-blown nonwovens for surgical mask production
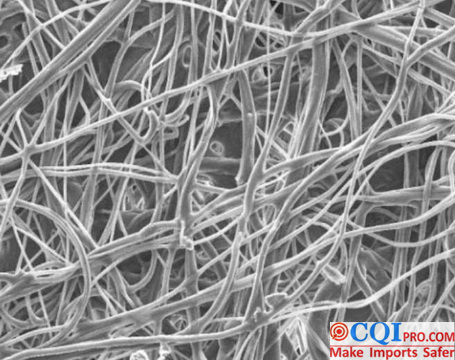
Melt Blowing belongs to the non-woven process of polymer extrusion. The principle of the process is to use high-speed hot air to draft the polymer melt trickle extruded from the die spinneret orifice, thus forming ultra-fine fibers and agglomerating them on the screen or roller, and adhering them to form non-woven fabric. Meltblown materials are non-woven fabrics produced by melt-blown method with thicker thickness by hot melting of their own fibers. The fibers are randomly and cross-arranged, forming a multi-curved channel structure of melt-blown materials, so that particles (new coronavirus) will collide with the fibers and be retained.
The filtration mechanism of medical mask is Brownian diffusion, interception, inertial collision, gravity sedimentation and electrostatic adsorption. The first four are all physical factors, i.e. the natural characteristics of non-woven fabrics produced by melt blowing, with a filterability of about 35%; this is not up to the requirements of surgical masks. We need to electretize the materials, charge the fibers, and electro statically capture the aerosol where the new coronavirus is located.
3. Stationary treatment
Electrostatic adsorption is the capture of new coronavirus droplets (aerosols) through the coulomb force of charged fibers. The principle is to make the surface of the filter material more open, and the ability to capture particles can be snatched, while the charge density increases, and the adsorption and polarization effects on particles are stronger. Therefore, the meltblown non-woven fabric filter material of the filter layer must undergo electrifying treatment to achieve 95% filterability without changing the respiratory resistance, and to effectively prevent viruses.
The mask should ensure comfortable ventilation while achieving the blocking effect. The suction resistance of masks is generally not more than 343.2 Pascals (Pa), while that of civil masks is less than 135 Pascals (Pa).The electret treatment can greatly improve the filtration efficiency without changing respiratory resistance, and the higher the electret voltage, the higher the material filtration efficiency.
4. Surgical mask production Line
The problem of raw materials has been solved, then mask production is just a matter of organizing workers and materials to maintain the production line capacity. This is especially fast. It’s all automated production lines.

A small mask factory may need 10 people, and the equipment includes mask forming machine, mask pressing machine, mask edge trimmer, breather valve punching machine, nose bridge line bonding machine, ear band spot welding machine, breather valve welding machine, etc.
Can keep doing quality inspection really improve the quality of products? This is of course, surgical mask production also need quality inspection. For example, is the nose bridge at the upper part of the mask correctly positioned? In this way, the mask will be tightly sealed. Otherwise, without this structure, the air leakage will be serious, which will affect the protection effect. In addition, we need to pull the ear cord to test or arrange the mask to avoid the occurrence of ear cord not being sewn firmly and symmetrically or whether there is appearance defect on the surface. All these belong to mask inspection.
5. Ethylene oxide disinfection
If it is an ordinary mask, it does not need disinfection, but medical use requires manufacturers to use ethylene oxide (EO) disinfection cabinet for disinfection. The mask was placed in an environment of 400mg/L ethylene oxide. Alkylation was applied to hydroxyl groups to inactivate micro-biological macromolecules and achieve the purpose of sterilization.
However, ethylene oxide is not only flammable and explosive, but also toxic to human body. Therefore, it needs to be allowed to stand for 7 days after disinfection for analysis. Only after EO residue is lower than the required value can it be packaged and delivered to medical personnel for use.
Only surgical mask production according to the above process can be qualified, safe and can be used to isolate the spread of new corona virus: Use high melt-blown nonwoven fabrics as the filter materials, undergo electrets treatment to increase electrostatic adsorption, be disinfected with ethylene oxide after production, and be allowed to stand for 7 days to analyze ethylene oxide.
At present, the Industrial and Commercial Departments all over China have also been making great efforts to crack down on fake masks. The kind of inferior mask that breaks at a single blow, using only one layer of non-woven fabric, manufacturers are serious cheating on materials and workmanship. The factory of surgical mask production , need to have “medical equipment business license”! Therefore, as a third-party inspection company, if Client need Chinese medical masks, we would suggest the guests to carry out factory audit at the factory first, ensure the mask factory has “Medical Device Business Enterprise License”. Secondly, mask manufacturers need to send masks to the Quality Supervision Bureau for LAB testing, meeting the requirements of GB18279.1-2015 and ISO11135:2014, because ethylene oxide is also a carcinogen.
CQI5 is committed to providing importers worldwide with product quality inspection services that far exceed those of our peers. If you are planning to import or have imported from China or Southeast Asian countries, please contact us cs’@’cqipro.com to learn more about how we can make your imports safer.
This article is an original article for CQI Inspection, who is committed to providing high-quality product inspection technology and know-how sharing for global importers and retailers to make imports safer.
All rights reserved. The contents of this website provided by CQI Inspection may not be reproduced or used without express permission.
For reprint, please contact with CQI Inspection, thank you.





